Abstract
Background: Immune dysregulation is a hallmark of chronic lymphocytic leukemia (CLL) and often leads to hypogammaglobulinemia and frequent infections. Treatment with anti-CD20 antibodies (Abs) such as rituximab (R) is a known risk factor for hypogammaglobulinemia. Despite the widespread use of anti-CD20 Abs in combination with venetoclax (Ven), a B cell lymphoma 2 inhibitor, little has been reported on the long-term impact of this therapy on B cell recovery. We report long-term immune changes post treatment in patients (pts) with relapsed/refractory CLL in the MURANO phase 3 trial (NCT02005471) who completed the full VenR course without progressive disease (PD).
Methods: Overall, 389 pts were randomized to either VenR (2 years of Ven with R for the first 6 months) or 6 months of bendamustine-R. Minimal residual disease (MRD) status has been previously reported (Kater et al. J Clin Oncol 2020). In the VenR arm, immunoglobulin (Ig) G, A and M, and T, B, and natural killer (NK) cells were measured centrally at baseline (BL), cycle 4 day 1 (C4D1), end of combination treatment (EOCT), end of treatment (EOT), and every 3 months for up to 3 years, then every 6 months until PD. This is a post hoc analysis among evaluable pts; statistics are descriptive only.
Results: In total, 130 pts completed 2 years of VenR without PD (27 pts were excluded from this analysis as they received Ig therapy during the study). BL characteristics amongst those included were consistent with the whole population. Median change from BL by visit (EOT, 12 months, and 24 months post EOT) was calculated among pts with paired evaluable BL and visit samples (Table 1).
IgG was significantly decreased at EOT vs BL (n=91; median change from BL, -5.9%). At 12 months (n=77; median change from BL, -4.8%) and 24 months (n=58; median change from BL, 1.1%) post treatment, IgG was not significantly different to BL levels. While there was no reduction in IgA from BL at EOT, at 12 and 24 months post treatment, IgA levels were significantly increased (median change from BL, 12.5% and 35.1%, respectively). IgM levels were significantly reduced at EOT vs BL (median change from BL, -32.3%), but returned to BL levels at 12 months post treatment (median change from BL, 11.1%). At 24 months post treatment, IgM had significantly increased by 49.5% compared with BL levels. There was no significant difference in median IgG, IgA and IgM levels at any time point amongst pts who achieved undetectable (u)MRD at EOT vs pts with MRD at EOT.
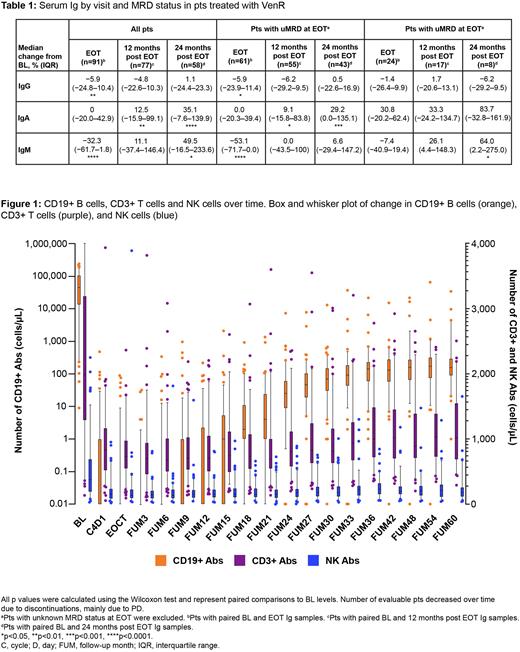
In pts who received VenR the overall infection rate was low; however, there was a non-statistically significant, but numerically higher rate of grade ≥3 infections occurring on treatment in those with uMRD at EOT compared with those who had MRD at EOT (15.2% [10/66] vs 7.1% [2/28]; p=0.5). T, B and NK cell data were available for all 130 pts. A depletion in CD19+ B cells was observed on treatment, which was more pronounced in pts with uMRD at EOT. After EOCT, B cell recovery started ~1 year later, and stabilized ~30 months after EOCT, regardless of EOT MRD status (Figure 1). Given the differences in disease burden at EOT, comparable CD19+ B cell recovery in pts with uMRD and MRD is likely to be representative of a larger relative proportion of normal B cells vs CLL cells. Additional analyses to test this are underway. At BL, total CD3+ T cells were increased (median [range], 1,991 [135-17,334]; reference values as previously reported, 700-2,100/µL [de Weerdt et al. Blood Adv 2019]). Though a reduction of CD3+ T cells was observed during combination treatment (C4D1), the number of CD3+ T cells were maintained within the reference range post treatment. A similar pattern was observed for NK cells. The median (range) CD4/CD8 ratio at BL was 1.10 (0.33-5.77) and was maintained throughout the study.
Conclusions: Overall, pts demonstrated immune recovery following fixed-duration treatment with VenR in this post hoc exploratory analysis. This is the first time that post-treatment serial Ig levels have been reported following VenR therapy. Recovery of IgG, IgA and IgM levels post treatment was observed regardless of EOT MRD status. While the overall incidence of grade ≥3 infections was low, the rate was numerically higher in pts with uMRD at EOT compared with pts with MRD. Additional analyses are underway to assess the correlation between infection and MRD state. A normalization of CD3+ T cells during and after VenR is consistent with previous reports.
Disclosures
Kater:Abbvie, Astra Zeneca, Janssen: Other: Speakers fee; Janssen, LAVA: Patents & Royalties: Pending; Astra Zeneca, BMS, Roche/Gennetech, Janssen, Abbvie, LAVA: Membership on an entity's Board of Directors or advisory committees; Abbvie, Astra Zeneca, BMS, Janssen, Roche/Genentech: Research Funding; Amsterdam UMC, University of Amsterdam: Current Employment. Eichhorst:Janssen, Roche, AbbVie, BeiGene, AstraZeneca, MSD: Speakers Bureau; Beigene: Other: Travel Support; Janssen, AbbVie, Lilly, AstraZeneca, BeiGene, MSD: Consultancy; Janssen, Roche, AbbVie, BeiGene, AstraZeneca: Research Funding. Owen:Incyte: Honoraria; GIlead: Honoraria; Roche: Honoraria; Merck: Honoraria; AstraZeneca: Honoraria; Janssen: Honoraria; Novartis: Honoraria; AbbVie: Honoraria; BeiGene: Honoraria. Jaeger:Miltenyi Biomedicine: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Gilead: Honoraria; BMS Celgene: Honoraria; Janssen: Consultancy, Honoraria; Abbvie: Honoraria; Sanofi: Honoraria; MSD: Honoraria; Beigene: Honoraria; Incyte: Honoraria; Acerta: Honoraria. Chyla:AbbVie: Current Employment, Current holder of stock options in a privately-held company. Lefebure:Roche: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Millen:Roche: Current Employment. Jiang:Roche/Genentech: Current Employment; Roche: Current equity holder in publicly-traded company. Thadani-Mulero:Roche Products Ltd: Current Employment; Roche Group: Current equity holder in publicly-traded company. Seymour:F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy; Celgene: Consultancy, Research Funding, Speakers Bureau; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genor Biopharma: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal